
Spectrum Saliva PCR (lab based processing) COVID-19 Collection Kit
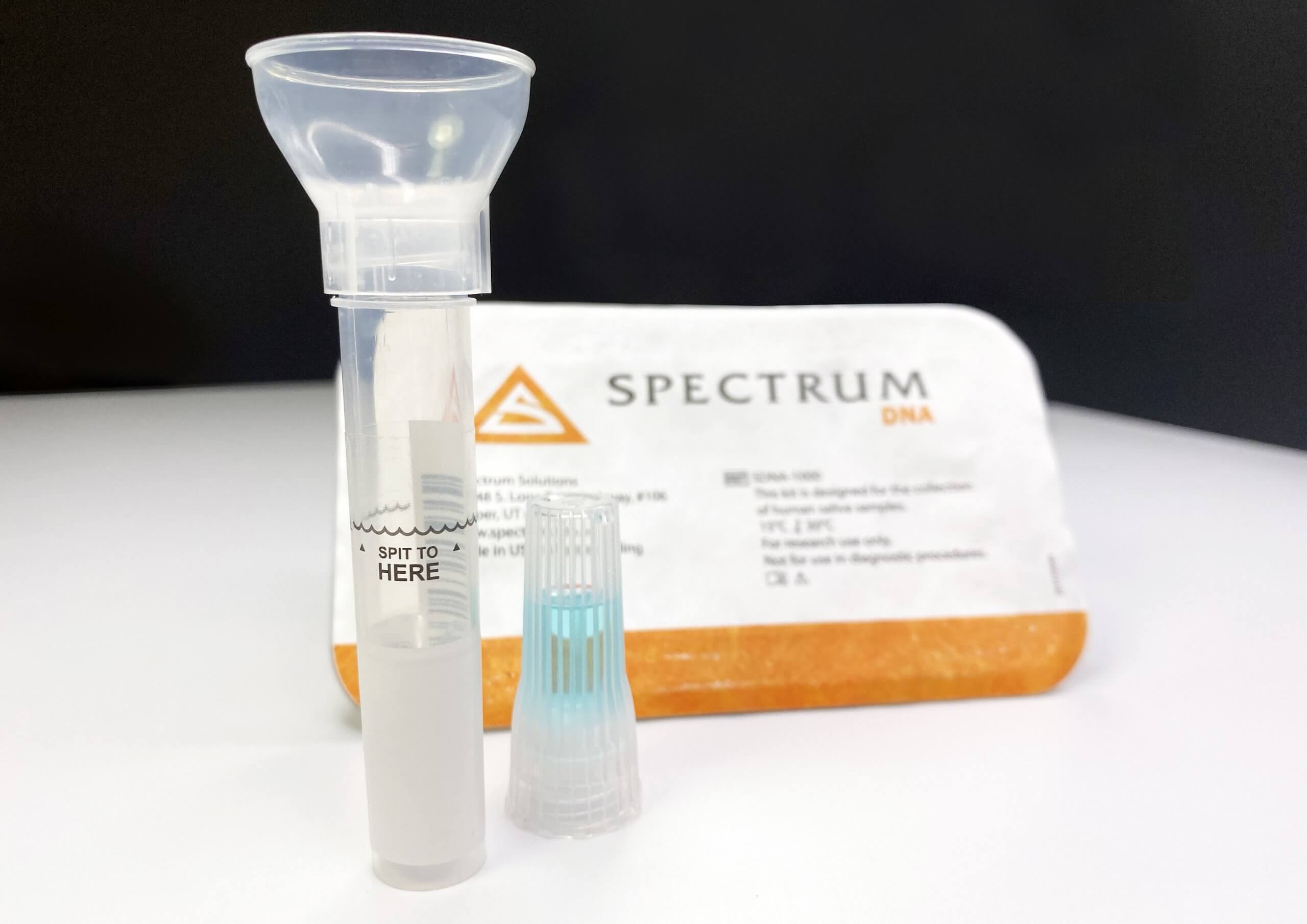
The Spectrum PCR COVID-19 collection kit is designed as a less intrusive alternative to the current swab collection method. “It supplants the need for the painful nasal swab process with a more accurate and pain-free saliva collection process. This easy-to-use option was engineered to eliminate user collection errors and provides a path for at-home sample self-collection that mitigates downstream exposure risks by delivering in-device viral inactivation. Offering the safest & most robust biomaterial for detecting COVID-19, the Spectrum SDNA saliva collection system provides over ten days of post-collection stability with no degradation in sample efficacy.”

The Spectrum Saliva Collection kit is being used by the Major League Baseball (MLB) in the United States as their official kit to test COVID-19 periodically.

Key Benefits
- SDNA-2000 and 3000
- Health Canada Approved
- This is a specimen collection kit to be used in conjunction with an authorized laboratory
- Only pain-free biosample collection option for COVID-19 testing
- Engineered to reduce self-collection error
- 100% inactivation of the live virus
- Single device preserves both DNA and fragile viral RNA transcripts
- Device EUA Authorization (EUA202432)
- Authorized by Health Canada with CE Mark
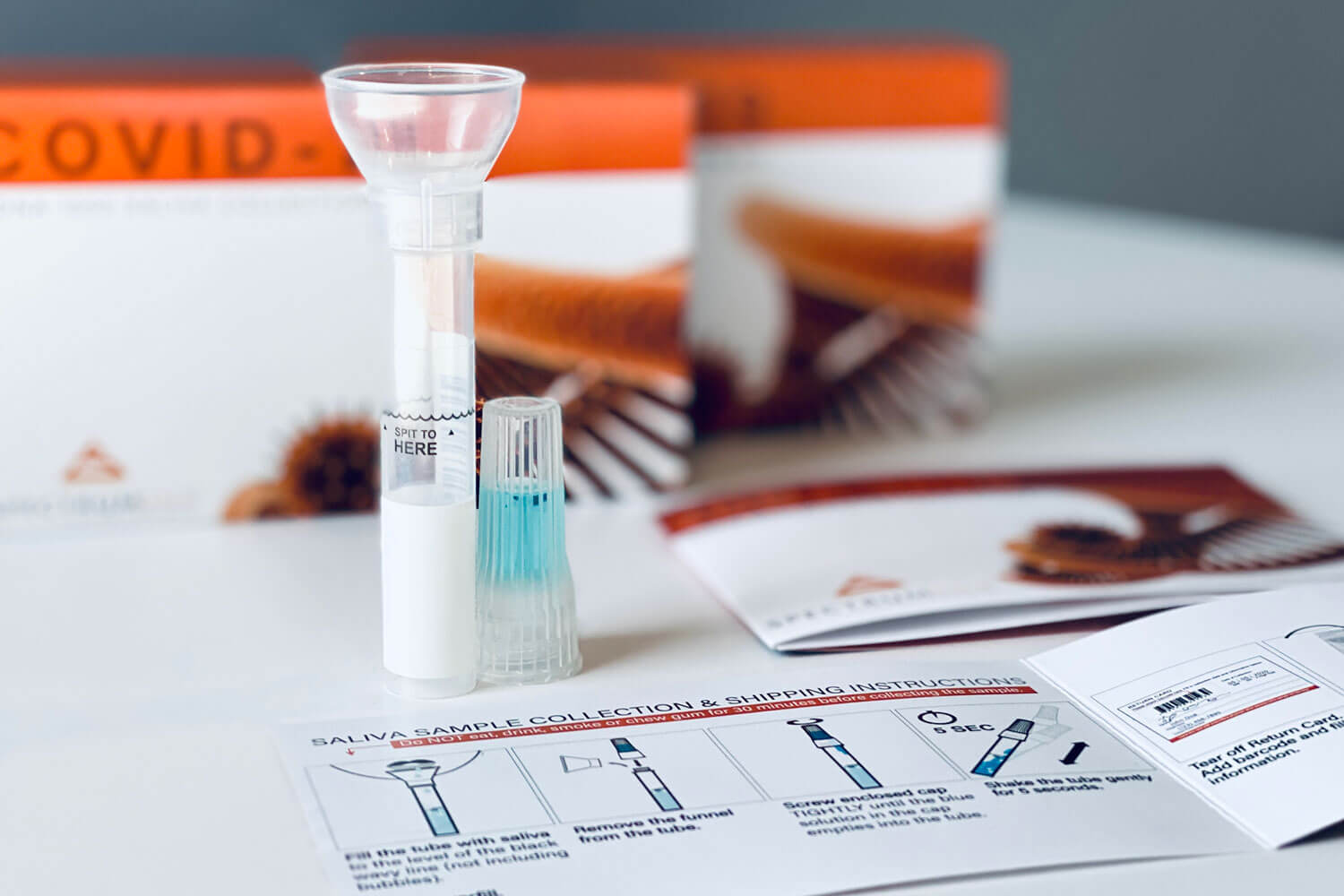
What is a Spectrum COVID Collection Kit?
First saliva collection kit FDA authorized (EUA202432) for COVID-19 diagnostic testing and analysis.
The SDNA saliva collection device has been engineered to lead the saliva collection market in molecular diagnostic applications. This self-contained saliva collection system provides critical sample consistency while suspending and neutralizing viral RNA transcripts post collection for sensitive and specific analysis, completely inactivating the live virus at ambient temperatures.
Spectrum’s technically-superior molecular diagnostic saliva collection systems provide long-term stability and ships with verified, unique barcode serialization for biosample digital chain-of-custody. Additionally, customized secondary packaging, kitting, and fulfillment options are available to solve any special project or testing workflow requirements.
Device Description
Features
- Simple: - Single device for DNA/viral RNA biosample collection, transportation, & storage
- Compatible: - Tube compatible with automated platforms & customizable for incremental efficiency improvements - Qualified commercial RNA extraction chemistries include Perkin Elmer, Thermo Fisher, Roche, Qiagen, & more
- Intuitive: - Intuitive 3-step design, proven to reduce customer collection errors
- Storage: - Post collection stability at room temperature for one year. Shelf life 24-months at room temperature for DNA
- Efficient: - System maintains biosample consistency. Significantly reduces costs associated with sample failures & recollection
- Shipping: - Standard mail at ambient temperatures. In device viral neutralization delivers shipping clearance with no biohazard UN3373 designation
- Solution: - Innovative, patented chemistry that preserves & protects both DNA/viral RNA transcripts, manages bacteria and mitigates any risk of infection throughout the testing process
- COVID-19: - First FDA EUA authorized saliva collection device for COVID-19 testing - First FDA EUA authorized device for direct- to-patient at-home sample self-collection - Delivers 100% inactivation of the live virus at ambient temperature
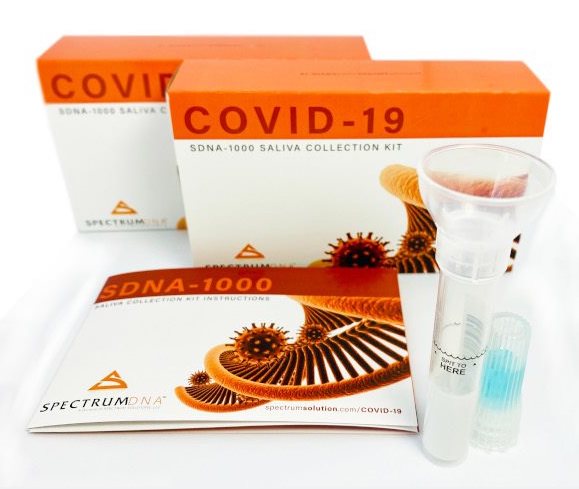
Saliva Collection Kits include
- Sealed blister pack with Spectrum Tyvek label
- Spit tube with attached funnel
- Unique barcode serialization for identification and sample chain-of-custody
- Device instructions for use in 8 languages
- Spectrum’s patented blue preservation chemistry filled cap
- Product shelf-life over 24 months
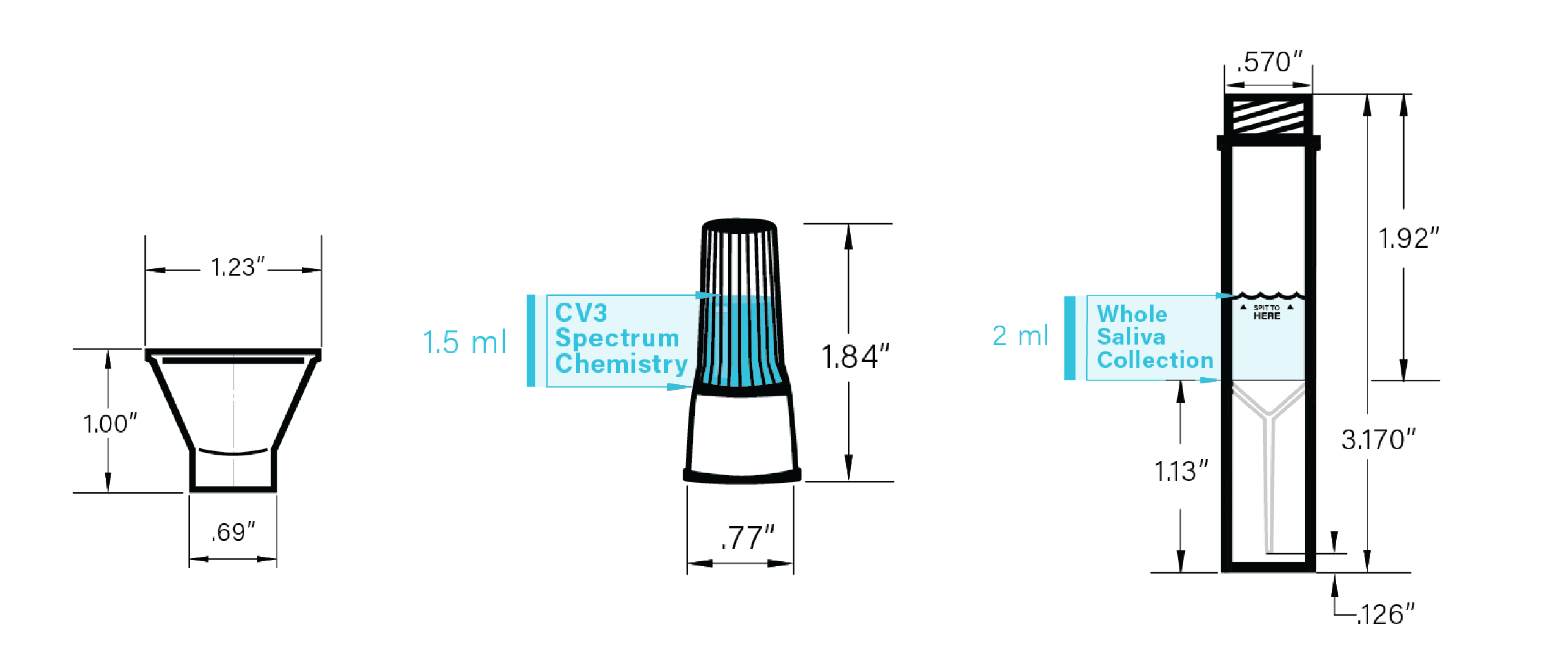
DEVICE SPECIFICATIONS
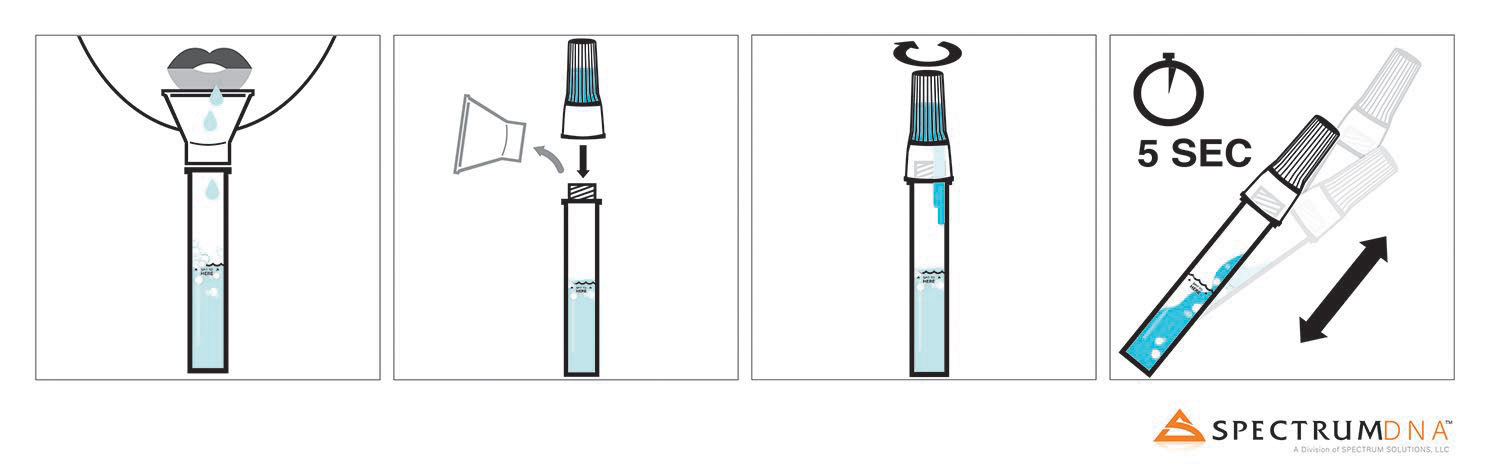
COLLECTION PROCESS
- 1. Fill the tube with saliva to the black wavy line (not including bubbles). Do not overfill.
- 2. Remove the funnel from the tube.
- 3. Screw enclosed cap TIGHTLY until the blue solution in the cap empties into the tube.
- 4. Shake the tube gently for 5 seconds.
Post-Collection Stability
Reference Links
Documents available upon request:
- Yale Study: Saliva is more sensitive for SARS-CoV-2 detection inCOVID-19 patients than nasopharyngeal swabs
- Spectrum Solutions White Paper
- Spectrum Sell Sheet SDNA 2000 & 3000
- Spectrum IFU Insert SDNA 2000 & 3000
- Spectrum Data Sheet SDNA 2000& 3000
- Spectrum IFU SDNA-3000 - Canada
*White Paper. The manufacturer is currently updating SDNA 2000 & SDNA 3000 documents.
Have Questions?
- If you're interested in having the Spectrum Saliva PCR Collection Kit for your business
- If you're a lab that wants to provide another option for PCR testing with a saliva kit
- If you want to set up a call with our team of experts for COVID-19 testing solutions for your business
