Regenerative Medicine
Regenerative medicine is an emerging field of research and clinical applications, focused on the repair, engineering, replacement or regeneration of cells, tissues or organs. The aim is to restore or establish normal function lost through congenital defects, disease or trauma. Technologies used include gene therapy, tissue engineering, reprogramming of cells and stem cell transplantation.
Of particular interest is the use of stem cell transplantation to repair injured tissue. There are two major types of stem cell transplants — autologous and allogeneic. An autologous stem cell transplant uses stem cells harvested from the same patient, whereas an allogeneic stem cell transplant uses stem cells harvested from another person.2 Autologous transplants have significant advantages over allogeneic transplants because they do not trigger immune rejection, eliminating the need for post-transplant immunosuppression.
The Science of Stem Cells
Bone Marrow-derived Mesenchymal Stem Cells (MSCs)
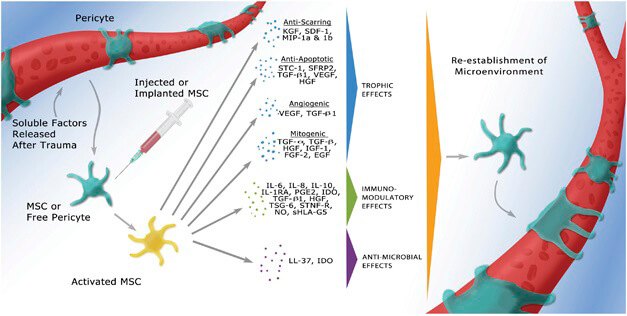
Mesenchymal stem cells (MSCs) are non-hematopoietic stromal cells present in many tissues.3 Their properties vary depending on the source from which they are harvested, but those found in the bone marrow develop into cells that form structural tissues like bone and connective tissue.3
MSCs from the bone marrow can be used to reconstitute bone, cartilage, muscle, ligament, tendon and fat.4 Autologous transplantation with MSCs offers a versatile approach to tissue repair.3 MSCs can contribute to the regeneration of various tissues and can therefore be used as patient-specific treatments for tissue injuries.3,4 Unlike traditional pharmaceutical agents, which are typically delivered systemically, this approach recapitulates the normal healing process. Transplanted MSCs secrete bioactive molecules locally, in the area where they are needed to help tissue repair, so they have no direct effect outside that microenvironment.3
The versatility of MSCs leads the way for a variety of pathologies in branches of medicine such as cardiology,5-8soft-tissue trauma,9,10 neurological disorders11,12 and spinal cord injury.13 They also have a wide range of potential uses in orthopedics and sports medicine.14-17
Benefits of Platelet-rich Plasma
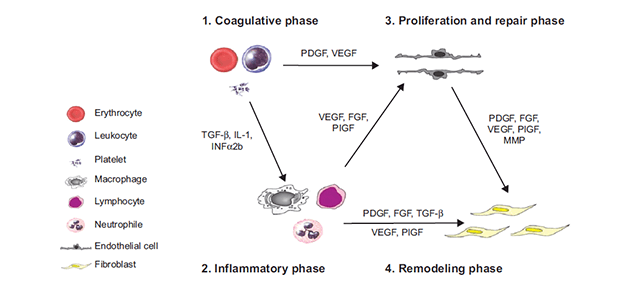
Autologous blood, which is obtained from the same patient, can be separated and used to treat tissue damage. Centrifugation of autologous whole blood separates it into red blood cells, white blood cells, and plasma containing platelets. The plasma obtained is a highly-concentrated source of platelets that can be used for tissue repair.16
Autologous platelets are used in regenerative medicine to help repair damaged tissue. Circulating platelets contain growth factors that are thought to enhance the healing properties of soft tissues. These growth factors are important for cell proliferation, differentiation and neovascularization. PRP also contains proteins that help recruit MSCs and fibroblasts to the repair site.16 PRP contains growth factors that enhance tissue healing and can be used to help heal acute and chronic injuries, including soft tissue injuries, cartilage defects and osteoarthritis.16 PRP may also be useful for a variety of dermatologic and aesthetic applications, including treatment of hair loss (alopecia), skin rejuvenation and filling, management of stretch marks (striae distensae) and minimization of scars on the skin.17
References
1. Mason C & Dunnill P. A brief definition of regenerative medicine. Regenerative Medicine 2008;3(1):1–5.
2. Zakrzewski W et al. Stem cells: past, present, and future. Stem Cell Research & Therapy 2019;10(1):68.
3. Murphy MB et al. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine 2013;45:e54.
4. Chamberlain G et al. Concise Review: Mesenchymal Stem Cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749.
5. Frantz S et al. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res 2009; 81: 474–481.
6. van Ramshorst J et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA 2009; 301: 1997–2004.
7. Williams AR et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 2011; 108: 792–796.
8. Tateishi-Yuyama E et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 2002; 360: 427–435.
9. Rasulov MF et al. First experience in the use of bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med 2005; 139: 141–144.
10. Bey E et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen; 18: 50–58.
11. Yamout B et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol 2010; 227: 185–189.
12. Connick P et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 2012; 11: 150–156.
13. Park JH et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery 2012; 70: 1238–1247.
14. Hernigou P et al. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg 2005: 1430–1437.
15. Hernigou Pet al. Percutaneous implantation of autologous bone marrow osteoprogenitor cells as treatment of bone avascular necrosis related to sickle cell disease. Open Orthop J 2008; 2: 62–65.
16. Gobbi A & Fishman M. Platelet-rich plasma and bone marrow–derived mesenchymal stem cells in sports medicine. Sports Med Arthrosc Rev 2016;24:69–73.
17. Leo MS et al. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol 2015;14:315–323.
18. Improving patient outcomes following glaucoma surgery: state of the art and future perspectives. Clinical Ophthalmology 2014
